Audit MedTech
How to finally get management’s attention as a MedTech Quality Manager
… so you can effectively fulfill your role in Quality Management and Regulatory Affairs again.
Gain a clear, expert-validated understanding of your current QM challenges
Leverage our reputation to secure management buy-in
Obtain the budget you need to address regulatory issues
Low-risk approach: only a one- or two-day workshop required

You strive to do your best every day.
As a quality assurance professional, you recognize internal issues and want to address them. Yet, management often doesn’t listen or take these concerns seriously.
The good news is: We understand what you need.
You need an external expert who can clearly communicate your current situation to management through an audit, highlighting both the existence of problems and the need for solutions.
Our consultants are experienced partners who understand the challenges you face, act as your sparring partner and work alongside you to tackle those challenges.
When asked, “Have you helped clients solve similar problems before?”, we happily share our experience and best practices from previous quality management projects. One thing is certain:
Your quality management will get back on track!
Components of an Internal Audit
We review all outstanding issues and provide a customized action plan, guiding you and your organization step by step toward your goals.
We conduct a comprehensive analysis of your entire quality management system.
We verify that all documentation meets the relevant standards.
We examine every aspect of your processes to identify potential issues.
We ensure that the validation of your machine processes is thoroughly documented.
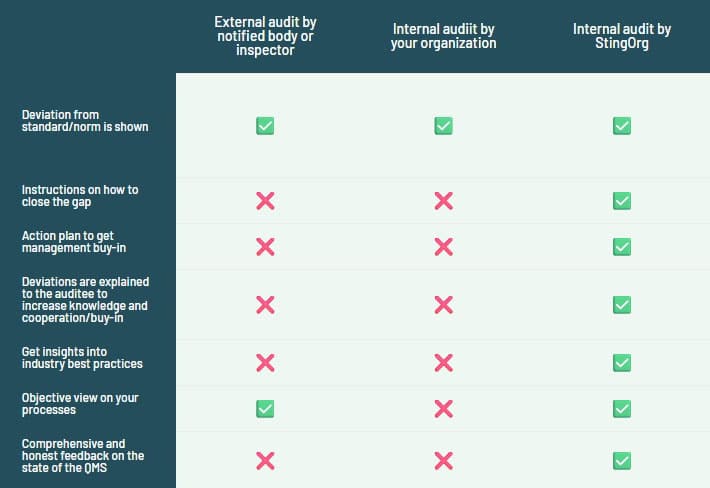

Why an audit makes sense for you
The audit checks whether your processes and products comply with the applicable standards and regulations and also helps to improve the quality of your products and processes.
The internal audit is carried out by an independent StingOrg consultant who is not part of your company. This ensures that the audit is conducted objectively and impartially.
With the help of the audit, we identify possible risks and minimize them.
We conduct an internal audit on the basis of these regulations and standards
ISO 13485
ISO 13485 is a standard that defines requirements for a quality management system for medical device manufacturers. The core goal is to develop safe and functional products.
EU MDR (2017/745)
The European MDR (Medical Device Regulation) must be adhered to manufacturers who want to place medical devices on the market in the EU. All medical devices must receive a unique identification, the UDI (Unique Device Identification). This means that the products must be registered in the EUDAMED (European Database on Medical Devices). The MDR specifies precise requirements for the instructions for use, other accompanying materials and other labelling such as imprints and packaging.
EU IVDR (2017/746)
The IVDR (In Vitro Diagnostics Regulation) requires manufacturers to conduct clinical studies on performance and to demonstrate that the safety and performance of the devices are appropriate for the respective risk class of the device.
MDSAP
The MDSAP (Medical Device Single Audit Program) is a defined procedure that the 5 initial participating countries (USA, Canada, Brazil, Japan and Australia) have developed and agreed to ensure harmonized procedures for inspections by their authorities and audits by approved certification bodies. An “MDSAP certification” simplifies the entry of products into the mentioned markets and is usually accepted as sufficient by the respective countries without an additional inspection by their responsible authority. Other countries have now joined this audit system or accept it. Within the EU, an exclusive MDSAP audit is not accepted as sufficient. MDSAP is based on ISO 13485:2016 and is supplemented by country-specific requirements. Further information and guidance documents on MDSAP are available free of charge on the homepage of the U.S. Food and Drug Administration (FDA).
QSR (21CFR…)
The QSR is an FDA regulation that defines the requirements for the quality management system of medical device manufacturers. The regulation specifies that medical device manufacturers must establish, implement, and maintain a quality management system to ensure that their products are safe and effective. The new regulation QMSR is published by FDA and will be mandatory in 2026.
ISO 14971
The ISO 14971 standard mainly refers to risk management for patients, users and other stakeholders, equipment and the environment. It specifies how medical device manufacturers must proceed to identify possible hazards and how to assess and calculate the risks of these hazards as well as the assessment of the effectiveness of measures used to control risks.
That’s why us!
- You not only receive a review of your current QMS’s status, but also an action plan to achieve or maintain certification
- We support you in every way to find sustainable solutions for and with you
- You benefit from many years of experience in a wide variety of organizations and best practices for almost every QM problem

Benefits of engaging StingOrg for internal audit
Finally consistent quality assurance
Independence through external view
End-to-end risk management
Ask a consultant, not a search engine
What is your current challenge in quality assurance?
Ask us a question – we will answer you immediately!
*Mandatory fields
Do you want to move
your business forward?
Let’s take the first steps together!

